Moderna Seeks FDA Authorize For Fourth Dose Of COVID-19 For Adults
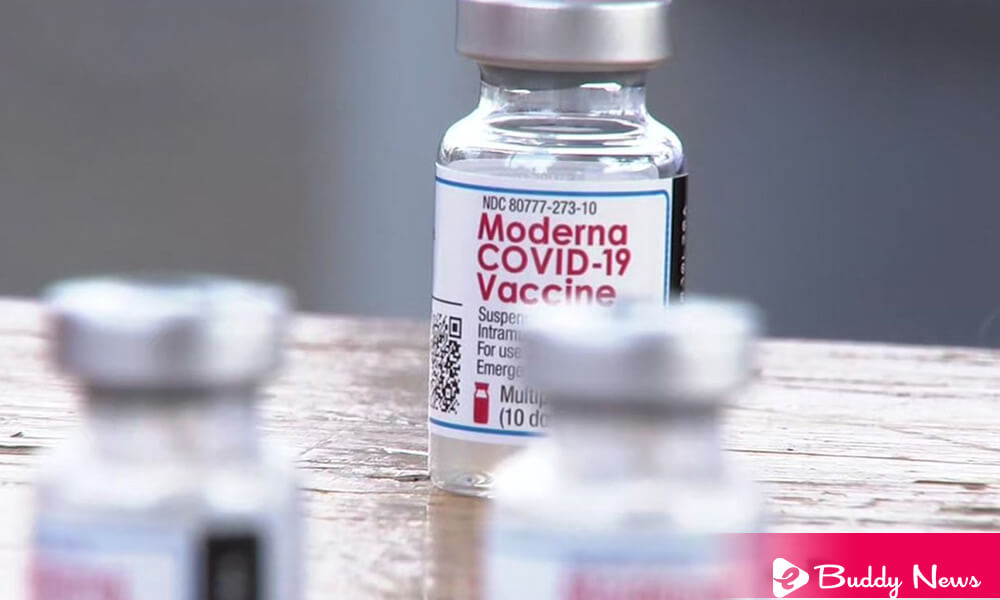
On Thursday, the pharmaceutical company Moderna seeks the US Food and Drug Administration (FDA) to authorize the fourth dose of its ‘Vaccine against COVID-19’ as a booster dose for all adults.
The same company also previously announced that it had begun clinical trials in the second phase of its study of a booster dose to fight the other variants of ‘Covid-19.’
The appeal is wider than the appeal of rival drug companies- ‘Pfizer’ before in the existing week asking the regulator to authorize a ‘booster dose’ for all the old age people.
The company said in a news release that Moderna seeks its ‘approval request’ to FDA for the fourth dose for all adults to give flexibility to the CDC and ‘health care providers.’ The statement is true to decide the reasonable use of a second booster dose of the ‘mRNA’ vaccine, including those at increased risk of contracting COVID-19 due to age or comorbidities.
The US officials have been laying the groundwork for giving additional booster doses to bolster the protection of vaccines against serious illness and death from ‘COVID-19.’
The White House also warned that it would need Congress to endorse more funds without delay for the ‘Federal Government’ to obtain more doses of vaccines against ‘COVID-19.’ It will facilitate either for ‘additional booster vaccines’ or for ‘variant immunizations.’